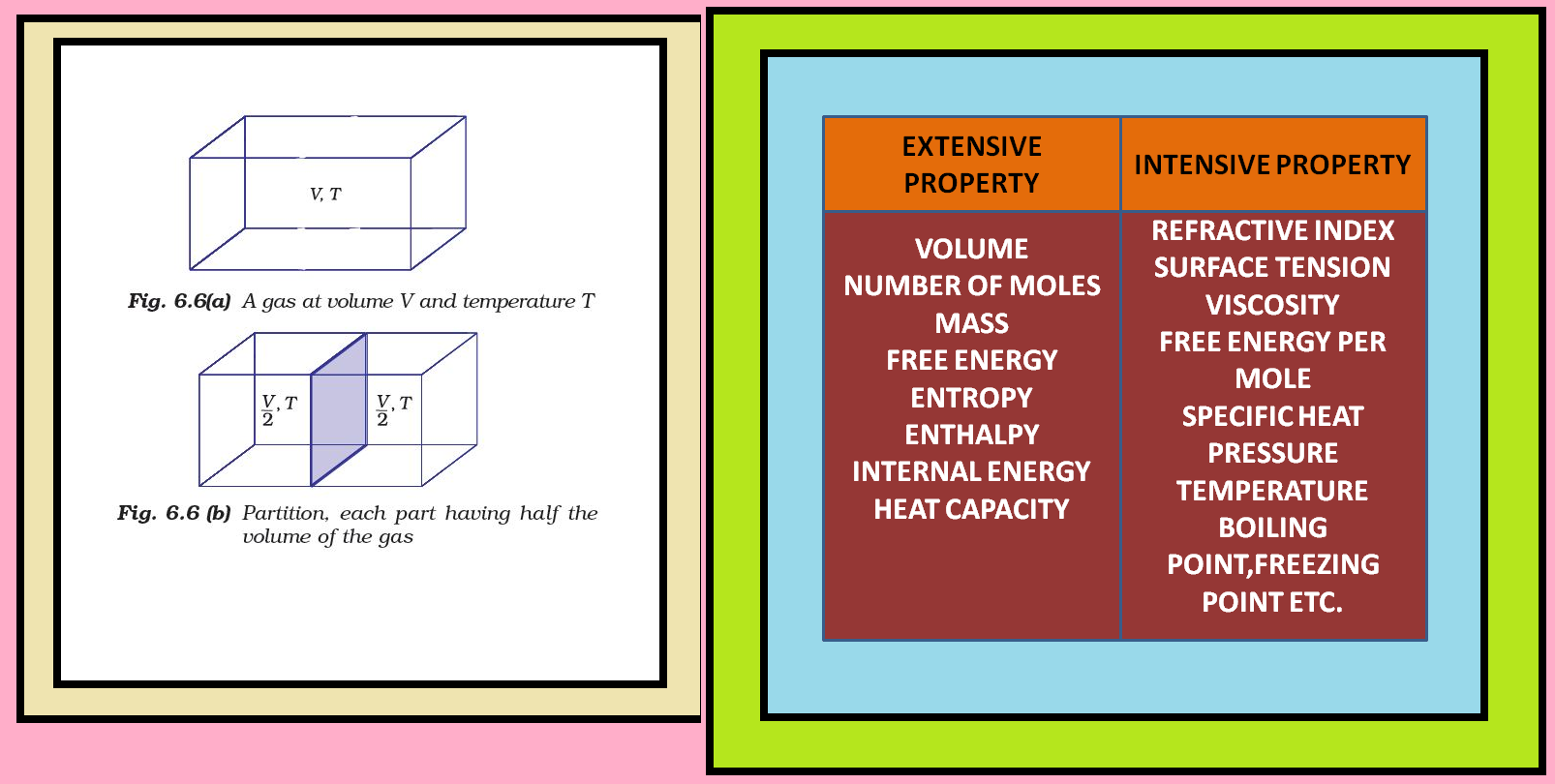
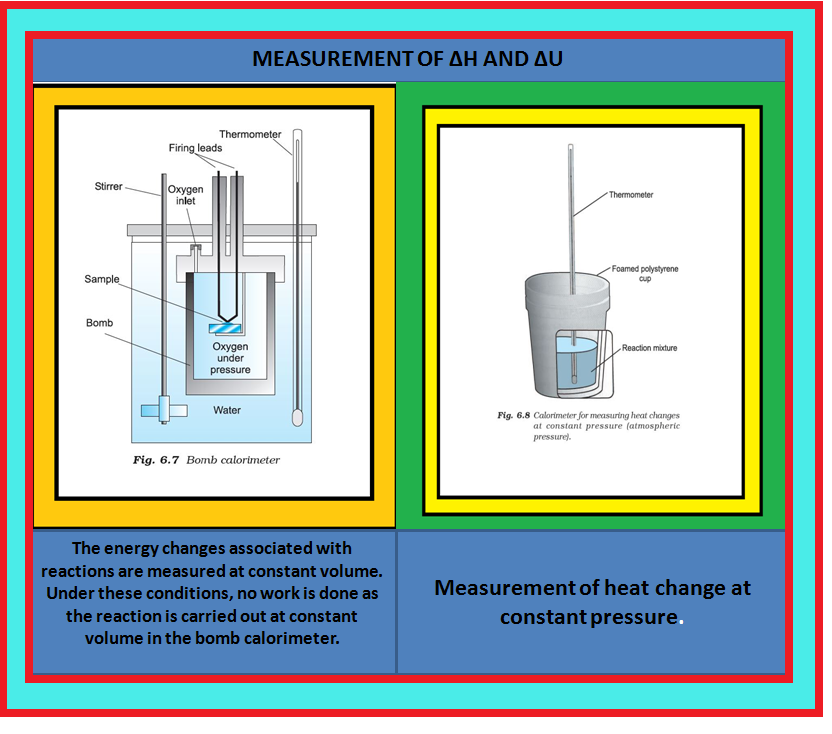
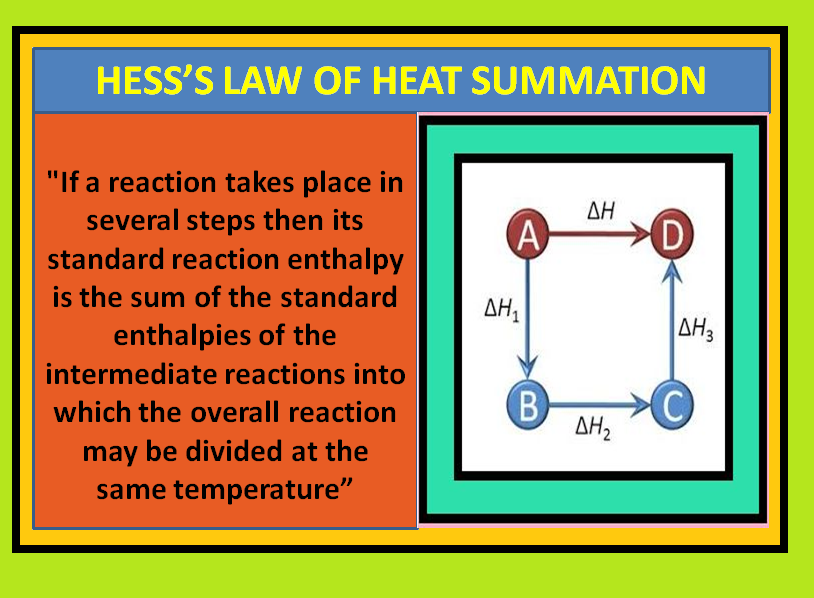
Thermodynamics
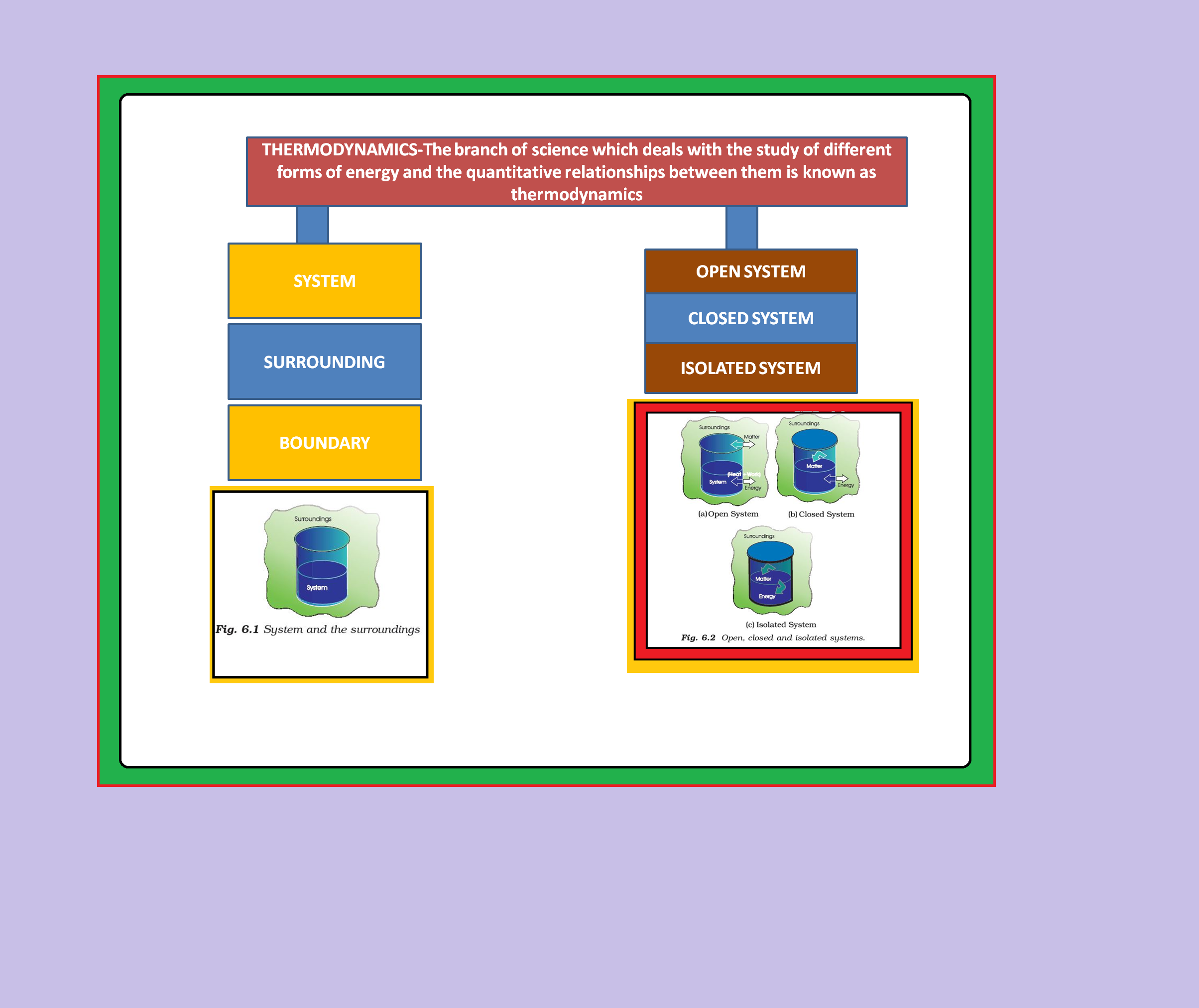
•`color{green}["Thermodynamics"]`:The branch of science which deals with the study of different forms of energy and the quantitative relationships between them is known as thermodynamics.
•`color{green}["System and surroundings"]`:A system in thermodynamics refers to that part of universe in which observations are made and remaining universe constitutes the surroundings.
•`color{green}["Boundary"]`:The wall that separates the system from the surroundings is called boundary. This is designed to allow us to control and keep track of all movements of matter and energy in or out of the system.
•`color{green}["Open system"]`: In an open system, there is exchange of energy and matter between system and surroundings
•`color{green}["Closed system"]`: In a closed system, there is no exchange of matter, but exchange of energy is possible between system and the surroundings.
•`color{green}["Isolated system"]`:In an isolated system, there is no exchange of energy or matter between the system and the surroundings.
•`color{green}["System and surroundings"]`:A system in thermodynamics refers to that part of universe in which observations are made and remaining universe constitutes the surroundings.
•`color{green}["Boundary"]`:The wall that separates the system from the surroundings is called boundary. This is designed to allow us to control and keep track of all movements of matter and energy in or out of the system.
•`color{green}["Open system"]`: In an open system, there is exchange of energy and matter between system and surroundings
•`color{green}["Closed system"]`: In a closed system, there is no exchange of matter, but exchange of energy is possible between system and the surroundings.
•`color{green}["Isolated system"]`:In an isolated system, there is no exchange of energy or matter between the system and the surroundings.